Novavax Covid Vaccine Composition
Importantly this vaccine works differently to the three approved vaccines and it has proven to be efficacious against two emerging variants of Covid-19 in the UK and South Africa. Novavax has now completed the submission of all modules required by MHRA for the regulatory review of NVX-CoV2373 the companys recombinant nanoparticle protein-based COVID-19 vaccine with Matrix.
Matrix-M adjuvant is a valuable component of vaccine development providing multiple immune system enhancements a well-understood mechanism of action and robust clinical experience where it was shown to be well tolerated in human studies to date.

Novavax covid vaccine composition
. This is the case for Novavax an American pharmaceutical company specializing in vaccines. Package ready for filing by the end of 2021. Novavax saponin-based Matrix-M improves immune responses and enables vaccine dose-sparing. Novavax is conducting late-stage clinical trials for NVX-CoV2373 its vaccine candidate against SARS-CoV-2 the virus that causes COVID-19.Novavax Vaccine getting ready to apply for full approval. Covid-19 Vaccine Novavax. Novavax at risk of losing time advantage once Covid-19 vaccine competitors flex production muscle. At the beginning of 2021 Australia agreed to purchase 51 million doses of a COVID-19 vaccine made by US biotech company Novavax.
Novavax submitted its first authorization filing for its COVID-19 vaccine in the UK. The Novavax vaccine NVX-CoV2373 contains purified pieces of the spike protein of SARS-CoV-2 the virus that causes COVID-19. Studies have shown the COVID-19 Novavax vaccine which is easy to store and transport to be 90 per cent effective against the virus. Canada committed to partnership with Novavax despite report of production problems with COVID-19 vaccine By Tonda MacCharles Ottawa Bureau Wed Oct.
Novavax announced Monday morning that its vaccine against Covid-19 proved 90 effective in preventing the disease in a large study group. And some continue to develop new ones or expand their offer. The trial achieved its primary endpoint in which NVX-CoV2373 Novavax recombinant nanoparticle protein-based COVID-19 vaccine demonstrated 100 protection against moderate and severe disease and 904 efficacy overall. 20 2021 timer 3 min.
In August 2020 Novavax is preparing the vaccine against Covid-19 the NVX-CoV2373. Novavax is firming as the vaccine the New Zealand Government will use for a programme of Covid-19 booster shots with a big shipment expected to arrive early next year. Wednesday and expects to follow suit in several other global markets in the coming weekThe US. Suspension for intramuscular injection Multi-dose vial 10 doses of 05 mL Record datetime of first use.
And its Food and Drug Administration FDA is not among those but CEO Stanley Erck said the company intends to have its US. The Maryland-based company Novavax has developed a protein-based coronavirus vaccine called NVX-CoV2373In March the company announced. Filing marks first protein-based COVID-19 vaccine submitted to MHRA for authorization. By Reynald Castaneda 21 Oct 2021 Last Updated October 21st 2021 1645 Competitor protein subunit Covid-19 vaccine manufacturers are slowly closing in at Novavaxs lead.
NanoFlu its quadrivalent influenza nanoparticle vaccine met all primary objectives in its pivotal Phase 3 clinical trial in older adults and will be advanced for regulatory submission. Novavaxs NVX-CoV2373 was thought to have an edge because it was the. All modules required for regulatory review including CMC data are now complete. CHATTANOOGA Tenn WDEF T he Novavax Covid vaccine.
Several vaccines against Covid-19 have already been on the market since the end of 2020 around the world. Jumped 80 in premarket trading on Wednesday after the company said it began submitting its experimental COVID-19 vaccine for authorization in the UK. PackageLabel Display Panel Vial Label. While Novavaxs NVAX COVID-19 vaccine shows potential delay in regulatory filings in both the United States and Europe does not bode well for the stock.
In June Novavax announced that its vaccine had proved about 90 percent effective against symptomatic COVID-19 in a study of nearly 30000 people in the US and Mexico. Full results from the PREVENT-19 pivotal Phase 3 trial of Novavax COVID-19 vaccine candidate have been posted to the medRxiv preprint server. Novavax is the fourth company to publish interim Phase III data for a Covid-19 vaccine. Shares of Novavax Inc fell 16 after a report from Politico said the company faces significant hurdles in proving it can manufacture its experimental COVID-19 vaccine.
Shares of Novavax Inc. Submission based on Phase 3 data from 45K patients demonstrating high efficacy and. Novavax Files for Authorization of its COVID-19 Vaccine in the United Kingdom.
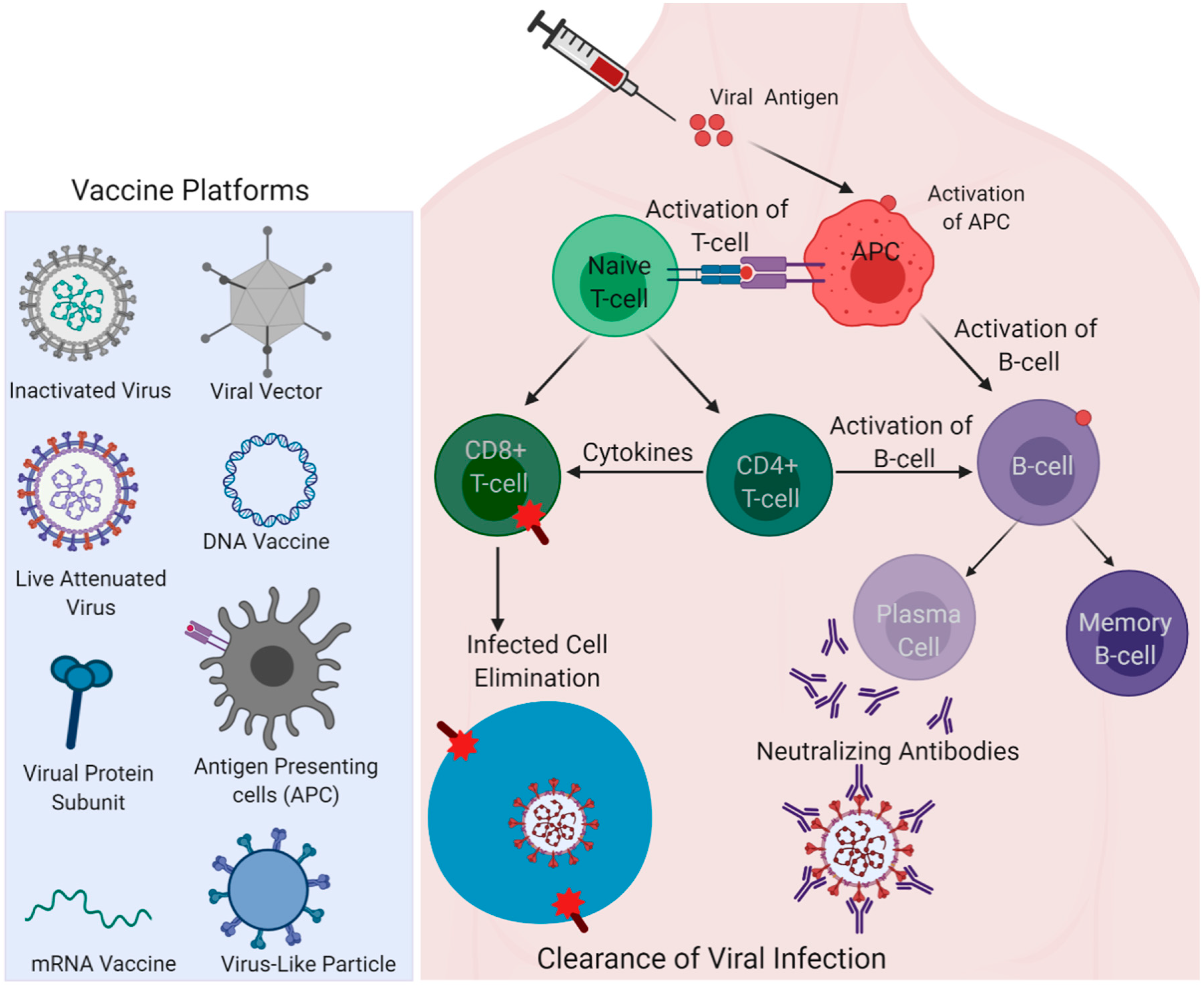
Vaccines Free Full Text Covid 19 Vaccines Currently Under Preclinical And Clinical Studies And Associated Antiviral Immune Response Html
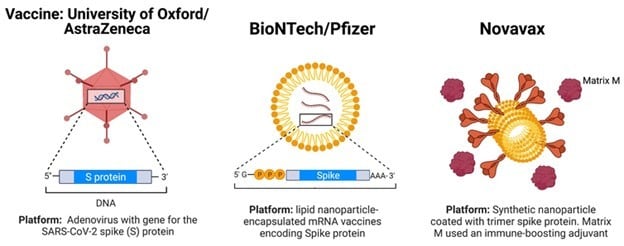
What Is Novavax Australia S Third Covid Vaccine Option The University Of Sydney

A Key Ingredient For Making A Covid 19 Vaccine Work Shots Health News Npr

Covid 19 Vaccine Platforms The Melbourne Vaccine Education Centre Mvec

Novavax Announces Further Delays For Regulatory Filings Of Covid 19 Vaccine Pmlive

Novavax Covid 19 Vaccine Nvx Cov2373 Everything You Need To Know Youtube
Novavax Says Its Covid Vaccine Is 90 Effective Plans Fda Submission In Q3


Comments
Post a Comment